For each of my Tech Talk Tuesdays, I will try to cover some concept in electronics or programming. If you have something you wish to see, please let me know in the comments! I'm open to suggestions, although it might take me a bit to get to them.
Batteries are fascinating devices that we often take for granted in our everyday lives. How often have you thrown out a AA alkaline cell and stopped to consider what actually happened inside that chemical-filled container to provide your remote control with electrical power? I know I never did (at least until now).
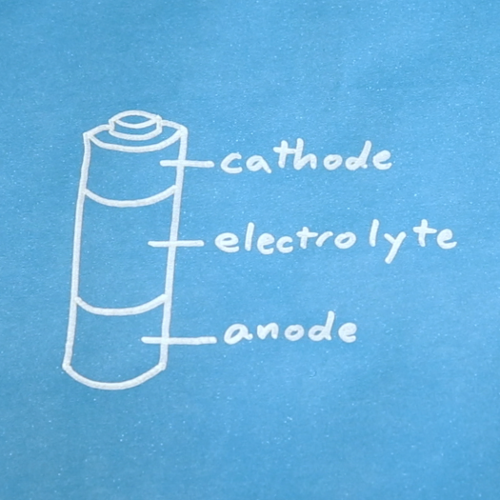
Batteries are made of three basic components:
- Anode: Electrons flow out of the anode in a battery, which means conventional "current" flows into the anode. The anode is marked as the negative (-) terminal on batteries.
- Cathode: Electrons flow into the cathode in a battery, which means conventional "current" flows out of the cathode. The cathode is marked as the positive (+) terminal on batteries.
- Electrolyte: The electrolyte is often a liquid or gel that reacts with the anode and cathode. It acts as an electrical insulator between the two but is capable of transporting ions.
Batteries can also have a separator to prevent the anode and cathode from touching, and most have some kind of casing to keep all the components contained.
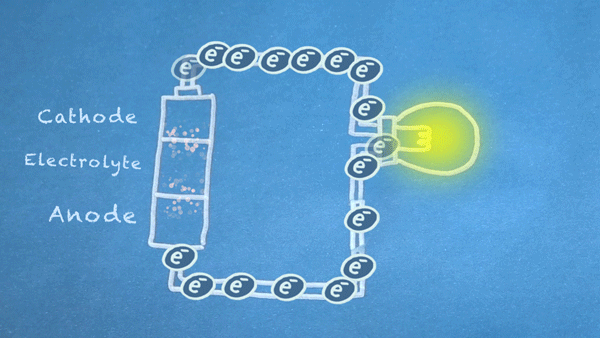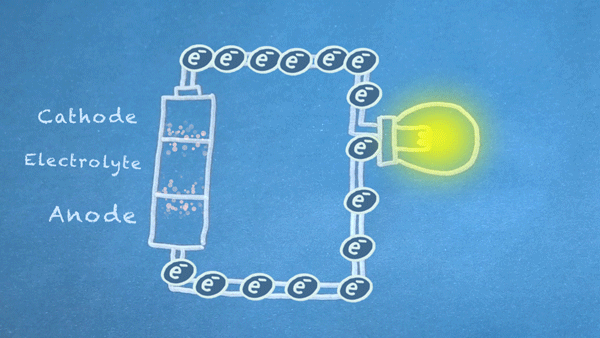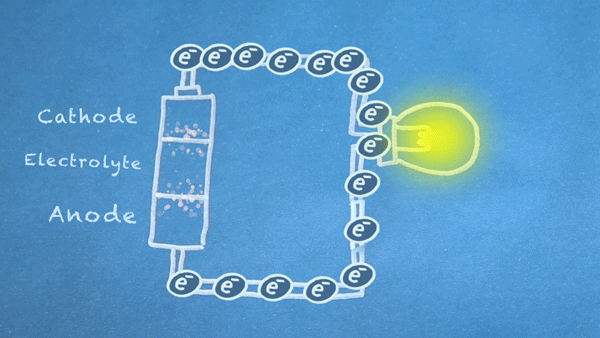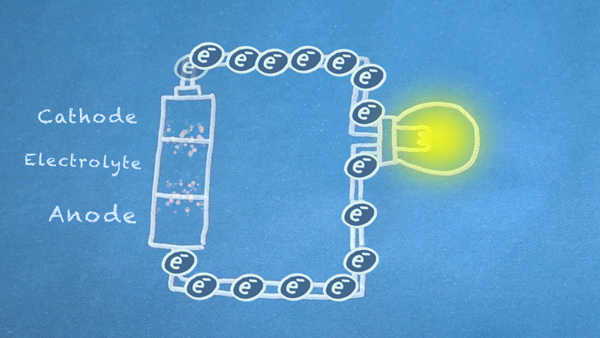
Batteries use chemical reactions to cause a flow of electrons. Two special reactions occur: oxidation and reduction. The first produces excess electrons, and the second requires extra electrons. The reactions happen inside the battery, and we harness the flow of electrons to perform work in our circuitry.
When an electrically conducting circuit connects the anode to the cathode, an oxidation reaction happens between the anode and the electrolyte. The extra electrons flow through our circuit toward the cathode.
In or around the cathode, the extra electrons are used in a reduction reaction. In some batteries, ions can also be produced and consumed during the oxidation and reduction reactions.
Some batteries need to be thrown out when the chemicals reach equilibrium. These batteries are known as primary cells.
Other types of batteries can be recharged, and these are called secondary cells.
If you would like to read a more thorough introduction to batteries, check out my What Is a Battery? tutorial:
What is a Battery?
May 3, 2016
An overview of the inner workings of a battery and how it was invented.







Hi Shawn,
Thanks for the great tutorial on batteries!
I wanted to add something from my own experience. The standard 9V alkaline (and maybe some of the other chemistries, not sure) can sometimes be comprised of 6 "AAAA" cells. There like AA or AAA cells, but slightly shorter and thinner. I had a device once (I think it was a cheapo flashlight) that ran on a AAAA cell, and when it died I pulled apart a 9V battery (energizer or Duracell, I think) and presto! 6 more cells.
I've also used them in projects where space was just a little bit too tight for AAAs.
I've seen AAAA before - where can you find them? Sadly, our 9V batteries aren't made of them. I tore one apart for a video, and found it was 6 custom cells stacked on top of each other (a still from the video can be found here).
I'm not sure what brand the 9V was, it was a long time ago. Maybe just get a couple different brands and pull them apart? I'm thinking the NiMH ones might be more likely to be AAAAs.
Digikey has Energizer brand ones here. Not very cheap, but if you need them.... :)
Most of the pharmacies near me have them (I guess some hearing aids use AAAA?). Horrendously expensive, though. Had to look for some because my "active" tablet stylus uses them (it's a Dell). The Surface Pro 4 stylus uses them too.
Thanks! Looks like bulk is the way to go if I need some :)
I have some SmartThings sensors that need AAAA batteries and I bought some off Amazon. 14 pack for about $8. http://www.amazon.com/Energizer-Alkaline-Batteries-Streamlight-Flashlights/dp/B000UUMQKW?ie=UTF8&psc=1&redirect=true&ref_=oh_aui_search_detailpage
Now prepare for the Conventional Versus Electron Flow arguments, and don't forget to put the Sealed Lead Acid battery back in the Emergency Lights. ;)
I did my best to clarify the conventional vs electron flow, but I know that it always causes confusion. Don't worry, I got the SLA from Nick. Not sure where he got it from, though :)